Drug Impurities Reference Standards
Showing 1631–1640 of 1775 results
-
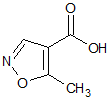
Leflunomide EP Impurity D
- Product Number CT-01110-912
- Parent Drug Leflunomide
- CAS Number 42831-50-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
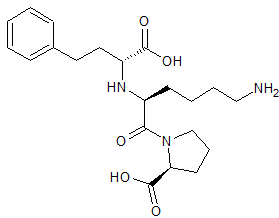
Lisinopril EP Impurity E
- Product Number CT-01110-934
- Parent Drug Lisinopril
- CAS Number 85955-59-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
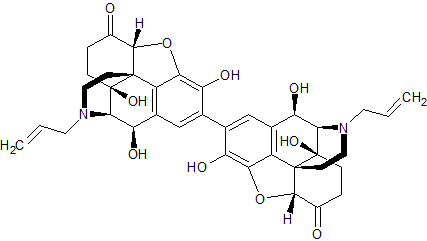
Naloxone Hydrochloride EP Impurity E
- Product Number CT-01110-843
- Parent Drug Naloxone
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
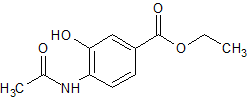
Oseltamivir EP Impurity D
- Product Number CT-01110-962
- Parent Drug Oseltamivir
- CAS Number 1346604-18-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Pioglitazone Impurity 6
- Product Number CT-01110-715
- Parent Drug Pioglitazone
- CAS Number 144809-28-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
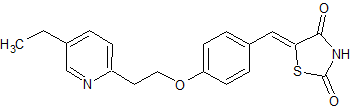
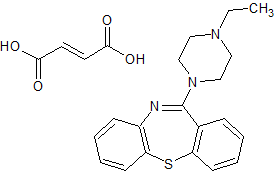
Quetiapine EP Impurity P Fumarate
- Product Number CT-01110-592
- Parent Drug Quetiapine
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
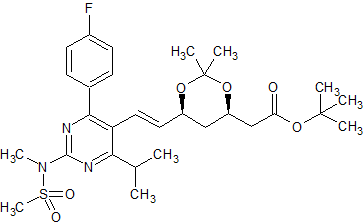
Rosuvastatin EP Impurity F
- Product Number CT-01110-497
- Parent Drug Rosuvastatin
- CAS Number 289042-12-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Urapidil Impurity 8
- Product Number CT-01110-1078
- Parent Drug Urapidil
- CAS Number 5464-78-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Tedizolid Impurity 10
- Product Number CT-01110-113
- Parent Drug Tedizolid
- CAS Number 1835340-19-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Empagliflozin Impurity 78
- Product Number CT-01110-155
- Parent Drug Empagliflozin
- CAS Number 874817-93-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options