Drug Impurities Reference Standards
Showing 1641–1650 of 1775 results
-
Tadalafil EP Impurity H
- Product Number CT-01110-276
- Parent Drug Tadalafil
- CAS Number 1346605-38-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
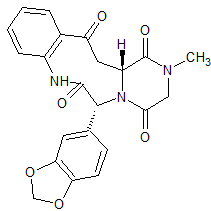
Alogliptin Impurity 1
- Product Number CT-01110-344
- Parent Drug Alogliptin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Sorafenib Impurity 2
- Product Number CT-01110-410
- Parent Drug Sorafenib
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
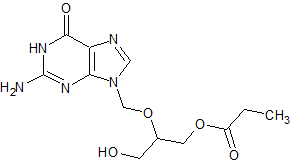
Ganciclovir EP Impurity B
- Product Number CT-01110-72
- Parent Drug Ganciclovir
- CAS Number 194159-18-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
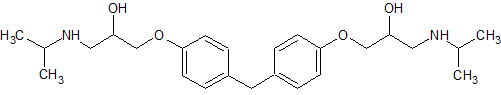
Bisoprolol EP Impurity C
- Product Number CT-01110-594
- Parent Drug Bisoprolol
- CAS Number 1797132-90-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Cefpodoxime Proxetil EP Impurity F
- Product Number CT-01110-879
- Parent Drug Cefpodoxime
- CAS Number 96680-30-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
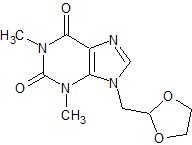
Doxofylline Impurity 3
- Product Number CT-01110-864
- Parent Drug Doxofylline
- CAS Number 1174289-18-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
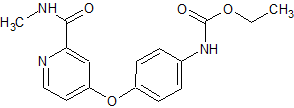
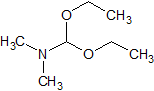
Flumazenil EP Impurity C
- Product Number CT-01110-786
- Parent Drug Flumazenil
- CAS Number 1188-33-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
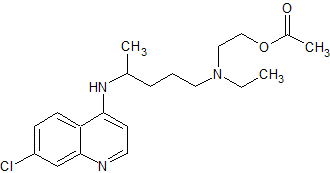
Hydroxychloroquine Impurity 7
- Product Number CT-01110-1024
- Parent Drug Hydroxychloroquine
- CAS Number 47493-14-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
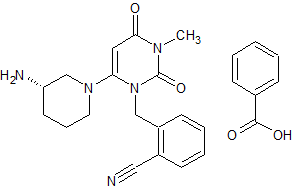
Leflunomide EP Impurity D
- Product Number CT-01110-912
- Parent Drug Leflunomide
- CAS Number 42831-50-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options