Drug Impurities Reference Standards
Showing 651–660 of 1775 results
-
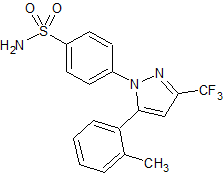
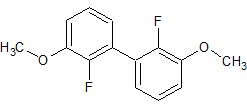
Celecoxib Impurity 8
- Product Number CT-01110-101
- Parent Drug Celecoxib
- CAS Number 170569-99-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
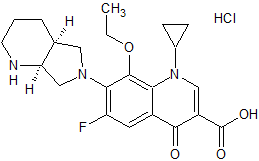
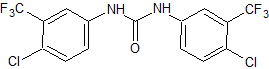
Moxifloxacin EP Impurity C
- Product Number CT-01110-192
- Parent Drug Moxifloxacin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
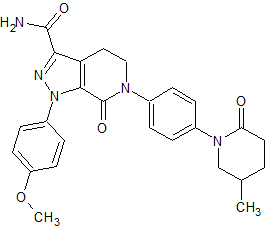
Apixaban Impurity 4 (BMS-728626-01)
- Product Number CT-01110-282
- Parent Drug Apixaban
- CAS Number 1686149-74-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
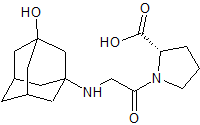
Vildagliptin Impurity 3
- Product Number CT-01110-348
- Parent Drug Vildagliptin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Sorafenib Impurity 9
- Product Number CT-01110-413
- Parent Drug Sorafenib
- CAS Number 370-50-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
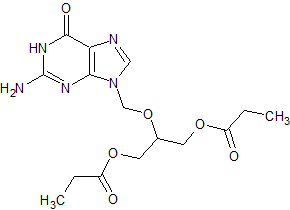
Ganciclovir EP Impurity I
- Product Number CT-01110-74
- Parent Drug Ganciclovir
- CAS Number 86357-20-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
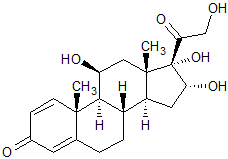
Budesonide EP Impurity A
- Product Number CT-01110-718
- Parent Drug Budesonide
- CAS Number 13951-70-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
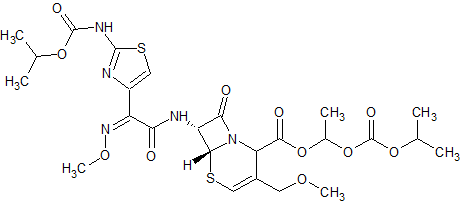
Cefpodoxime Proxetil Impurity 8
- Product Number CT-01110-881
- Parent Drug Cefpodoxime
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
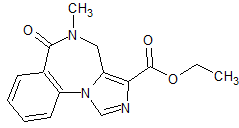
Elagolix Impurity 4
- Product Number CT-01110-1118
- Parent Drug Elagolix
- CAS Number 2316733-82-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Flumazenil EP Impurity E
- Product Number CT-01110-788
- Parent Drug Flumazenil
- CAS Number 78756-03-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options