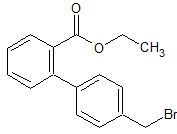
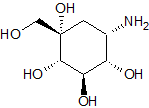
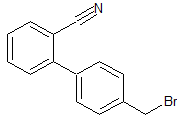
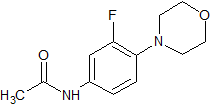
Drug Impurities Reference Standards
Showing 661–670 of 1775 results
-
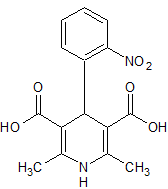
Nifendipine Impurity 7
- Product Number CT-01110-765
- Parent Drug Nifedipine
- CAS Number 74378-10-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
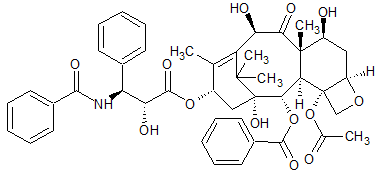
Paclitaxel EP Impurity G
- Product Number CT-01110-1034
- Parent Drug Paclitaxel
- CAS Number 78432-77-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
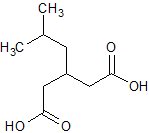
Pregabalin Impurity 2(3-IsobutylglutaricAcid)
- Product Number CT-01110-621
- Parent Drug Pregabalin
- CAS Number 75143-89-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
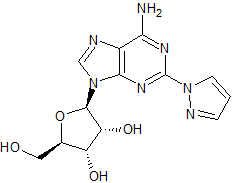
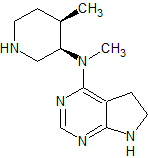
Regadenoson Impurity 5
- Product Number CT-01110-1099
- Parent Drug Regadenoson
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Telmisartan Impurity 3
- Product Number CT-01110-669
- Parent Drug Telmisartan
- CAS Number 133085-87-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Voglibose Impurity 3
- Product Number CT-01110-982
- Parent Drug Voglibose
- CAS Number 83465-22-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Valsartan ImpurIty 10
- Product Number CT-01110-129
- Parent Drug Valsartan
- CAS Number 114772-54-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
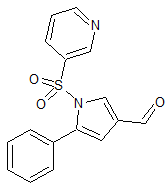
Linezolid Impurity 51
- Product Number CT-01110-178
- Parent Drug Linezolid
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Tofacitinib Impurity 13
- Product Number CT-01110-226
- Parent Drug Tofacitinib
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Vonoprazan Fumarate Impurity 40
- Product Number CT-01110-57
- Parent Drug Vonoprazan
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options